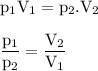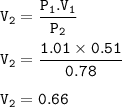Chemistry, 18.12.2020 01:00 animerocks07
A sealed balloon containing 0.51 L of air is carried from Toronto (P-1.01 atm) to Whistler (P=0.78 atm). What would the volume of the bag be in L) upon arrival in Whistler ?
Assume constant temperature and ideal gas behaviour.
Write your answer with 2 significant figures. Enter the number only - do not enter units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1

Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2
You know the right answer?
A sealed balloon containing 0.51 L of air is carried from Toronto (P-1.01 atm) to Whistler (P=0.78 a...
Questions

History, 26.07.2019 17:10

Mathematics, 26.07.2019 17:10

English, 26.07.2019 17:10


Mathematics, 26.07.2019 17:10