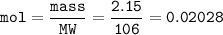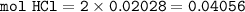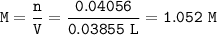Chemistry, 21.12.2020 16:00 ameliaxbowen7
If 38.55 mL of HCl is required to titrate 2.150 g of Na2CO3 according to the following equation, what is the molarity of the HCl solution? Na2CO3 + 2HCl (aq)→ 2NaCl (aq) + CO2 (g) + H20 (1)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
If 38.55 mL of HCl is required to titrate 2.150 g of Na2CO3 according to the following equation, wha...
Questions

English, 06.04.2021 22:10



Mathematics, 06.04.2021 22:10

English, 06.04.2021 22:10



History, 06.04.2021 22:10

English, 06.04.2021 22:10




English, 06.04.2021 22:10

History, 06.04.2021 22:10

Mathematics, 06.04.2021 22:10

Mathematics, 06.04.2021 22:10


Business, 06.04.2021 22:10


Mathematics, 06.04.2021 22:10