Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

You know the right answer?
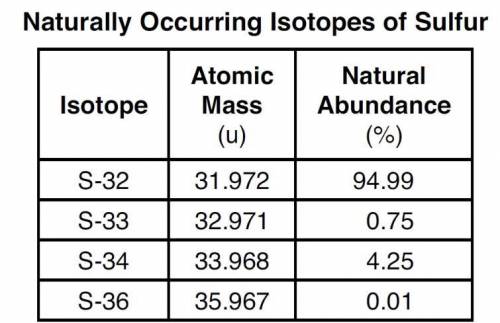
The four naturally occurring isotopes of sulfur are S-32, S-33, S-34, and S-36. The table below show...
Questions

Mathematics, 20.05.2020 06:57


Biology, 20.05.2020 06:57

Mathematics, 20.05.2020 06:57

Biology, 20.05.2020 06:57

History, 20.05.2020 06:57


Mathematics, 20.05.2020 06:57





Mathematics, 20.05.2020 06:57


Mathematics, 20.05.2020 06:57




Chemistry, 20.05.2020 06:57

Social Studies, 20.05.2020 06:57