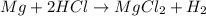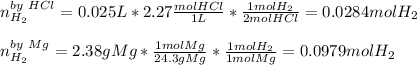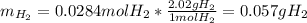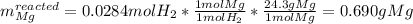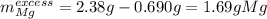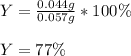1) When 2.38g of magnesium is added to 25.0cm of 2.27 M hydrochloric acid, hydrogen gas is released.
a) Determine the limiting reactant
b) Calculate the mass of hydrogen gas produced.
c) Calculate the mass of excess reactant remained at the end of reaction.
d) What is the percentage yield if 0.044g of hydrogen gas is obtained from the experiment?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
1) When 2.38g of magnesium is added to 25.0cm of 2.27 M hydrochloric acid, hydrogen gas is released....
Questions


Chemistry, 12.11.2020 14:00




Mathematics, 12.11.2020 14:00


Mathematics, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

Chemistry, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

History, 12.11.2020 14:00

Biology, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00

Mathematics, 12.11.2020 14:00