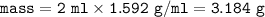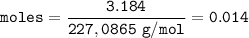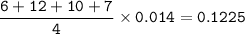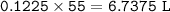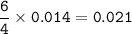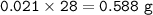Chemistry, 07.01.2021 09:40 LindaCat78
If a sample of nitroglycerin containing 2 mL (density = 1.592g/mL) is detonated, how many total moles of gas are produced? If each mole of gas occupies 55L under the conditions of the explosion, how many liters of gas are produced? How many grams of nitrogen gas are specifically produced?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
If a sample of nitroglycerin containing 2 mL (density = 1.592g/mL) is detonated, how many total mole...
Questions


Mathematics, 04.05.2021 23:40


History, 04.05.2021 23:40


English, 04.05.2021 23:40

Mathematics, 04.05.2021 23:40


Business, 04.05.2021 23:40



Mathematics, 04.05.2021 23:40






English, 04.05.2021 23:40

English, 04.05.2021 23:40

History, 04.05.2021 23:40