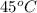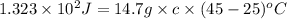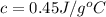An unknown substance has a mass of 14.7 g . when the substance absorbs 1.323×102 j of heat, the temperature of the substance is raised from 25.0 ∘c to 45.0 ∘c . what is the most likely identity of the substance?
a. aluminum 0.903
b. iron 0.449
c. water 4.184
d. silver 0.235
e. copper 0.385
f. ethanol 2.42

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1


Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
An unknown substance has a mass of 14.7 g . when the substance absorbs 1.323×102 j of heat, the temp...
Questions

Mathematics, 18.12.2020 17:10





World Languages, 18.12.2020 17:10


Mathematics, 18.12.2020 17:10












Mathematics, 18.12.2020 17:10

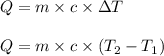
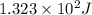
 = change in temperature
= change in temperature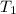 = initial temperature =
= initial temperature = 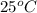
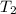 = final temperature =
= final temperature =