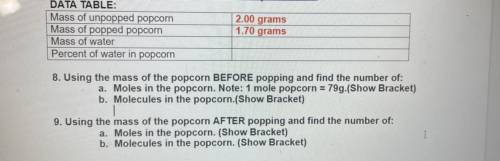
DATA TABLE:
Mass of unpopped popcorn
Mass of popped popcorn
Mass of water
Percent...

Chemistry, 08.01.2021 17:40 baaaaaaaagoat6222
DATA TABLE:
Mass of unpopped popcorn
Mass of popped popcorn
Mass of water
Percent of water in popcorn
2.00 grams
1.70 grams
8. Using the mass of the popcorn BEFORE popping and find the number of:
a. Moles in the popcorn. Note: 1 mole popcorn = 79g.(Show Bracket)
b. Molecules in the popcorn.(Show Bracket)
9. Using the mass of the popcorn AFTER popping and find the number of:
a. Moles in the popcorn. (Show Bracket)
b. Molecules in the popcorn. (Show Bracket)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Questions


History, 24.01.2020 12:31

History, 24.01.2020 12:31

Physics, 24.01.2020 12:31


Mathematics, 24.01.2020 12:31


English, 24.01.2020 12:31

Physics, 24.01.2020 12:31


Biology, 24.01.2020 12:31



English, 24.01.2020 12:31

Chemistry, 24.01.2020 12:31





Mathematics, 24.01.2020 12:31



