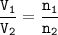Chemistry, 14.01.2021 20:10 tommyaberman
You want to know how many moles of gas your lungs can hold. You start off with a balloon that has 1.4 moles of gas and occupies 22.4L. You then blow all the air from your lungs into the balloon. The balloon now occupies 28.4L. How many moles of gas did you blow into the balloon.
A) 1.775 moles
B) .0013 moles
C) 43.56 moles
D) 2,654 moles

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
You want to know how many moles of gas your lungs can hold. You start off with a balloon that has 1....
Questions





Arts, 27.03.2020 01:51

Computers and Technology, 27.03.2020 01:51











Computers and Technology, 27.03.2020 01:52

Mathematics, 27.03.2020 01:52

Mathematics, 27.03.2020 01:52

Mathematics, 27.03.2020 01:52