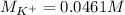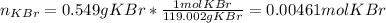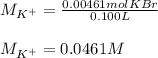Chemistry, 18.01.2021 14:00 makayyafreeman
Suppose of potassium bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the final molarity of potassium cation in the solution. You can assume the volume of the solution doesn't change when the potassium bromide is dissolved in it. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Suppose of potassium bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the...
Questions

Mathematics, 23.02.2021 23:40

Mathematics, 23.02.2021 23:40

Mathematics, 23.02.2021 23:40

Mathematics, 23.02.2021 23:40







Mathematics, 23.02.2021 23:40

Computers and Technology, 23.02.2021 23:40

English, 23.02.2021 23:40



Mathematics, 23.02.2021 23:40

English, 23.02.2021 23:40


History, 23.02.2021 23:40

Geography, 23.02.2021 23:40