
Chemistry, 18.01.2021 16:30 21teunissen149
A group 2 metal carbonate has a mass of 84 g/mol. Identify the group 2 metal X using its chemical formula. Please show your working out. number of moles in 0.3g of calcium carbonate.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
A group 2 metal carbonate has a mass of 84 g/mol. Identify the group 2 metal X using its chemical fo...
Questions

Mathematics, 31.12.2020 02:00



Mathematics, 31.12.2020 02:00

Mathematics, 31.12.2020 02:00



Spanish, 31.12.2020 02:00





English, 31.12.2020 02:00

Mathematics, 31.12.2020 02:00

Mathematics, 31.12.2020 02:00

English, 31.12.2020 02:00

Physics, 31.12.2020 02:00

Biology, 31.12.2020 02:00

Mathematics, 31.12.2020 02:00

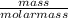
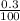 = 0.003mole
= 0.003mole


