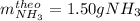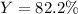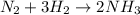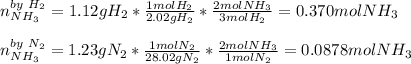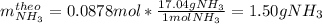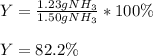Chemistry, 19.01.2021 18:50 jhernandezvaldez142
1.12g H2 is allowed to react with 9.60 g N2, producing 1.23 g NH3.
A. What is the theoretical yield in grams for this reaction under the given conditions?
B. What is the percent yield for this reaction under the given conditions?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
1.12g H2 is allowed to react with 9.60 g N2, producing 1.23 g NH3.
A. What is the theoretical yield...
Questions





Mathematics, 03.12.2020 23:50


Mathematics, 03.12.2020 23:50


Mathematics, 03.12.2020 23:50


Mathematics, 03.12.2020 23:50

Mathematics, 03.12.2020 23:50


Mathematics, 03.12.2020 23:50

Mathematics, 03.12.2020 23:50


Mathematics, 03.12.2020 23:50

History, 03.12.2020 23:50