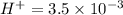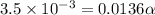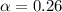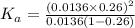Chemistry, 30.01.2020 14:54 LikeIke7105
Enough of a monoprotic acid is dissolved in water to produce a 0.0136 m solution. the ph of the resulting solution is 2.45. calculate the ka for the acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Enough of a monoprotic acid is dissolved in water to produce a 0.0136 m solution. the ph of the resu...
Questions

History, 24.08.2019 17:30


Chemistry, 24.08.2019 17:30



History, 24.08.2019 17:30



Mathematics, 24.08.2019 17:30


Mathematics, 24.08.2019 17:30

Social Studies, 24.08.2019 17:30



Mathematics, 24.08.2019 17:30


Physics, 24.08.2019 17:30

Mathematics, 24.08.2019 17:30

History, 24.08.2019 17:30

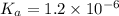
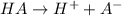
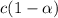
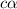
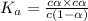
![[H^+]=c\alpha](/tpl/images/0486/2169/21a04.png)
![pH=-log[H^+]=2.45](/tpl/images/0486/2169/8dd78.png)