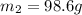Chemistry, 04.02.2020 11:54 tamikeen2243
To a sample of water at 23.4oc in a constant pressure calorimeter of negligible heat capacity is added a 12.1 g piece of aluminium whose temperature is 81.7oc. if the final temperature of water is 24.9 oc, calculate the mass of the water in the calorimeter. ans: 98.6g
-i know that the specific heat of aluminum is 0.900 j/g ‡ ác
- _t al is 24.9ác _ 81.7ác = _56.8ác
- _twater and _tcalorimeter are both 24.9ác _ 23.4ác = 1.5ác.
-the specific heat of water is 4.184 j/g ‡ ác.
but i tried using m=q/s_t. i'm really stuck, can anyone me?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
To a sample of water at 23.4oc in a constant pressure calorimeter of negligible heat capacity is add...
Questions


Chemistry, 04.09.2020 05:01



Mathematics, 04.09.2020 05:01

Mathematics, 04.09.2020 05:01



Mathematics, 04.09.2020 05:01

History, 04.09.2020 05:01



Mathematics, 04.09.2020 05:01


English, 04.09.2020 05:01



Mathematics, 04.09.2020 05:01

Geography, 04.09.2020 05:01

History, 04.09.2020 05:01

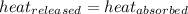
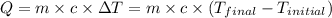
![-m_1\times c_1\times (T_{final}-T_1)=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0500/2981/27fc4.png) .................(1)
.................(1)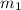 = mass of aluminium = 12.1 g
= mass of aluminium = 12.1 g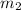 = mass of water = ?
= mass of water = ?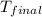 = final temperature =
= final temperature = 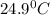
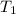 = temperature of aluminium =
= temperature of aluminium = 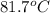
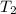 = temperature of water =
= temperature of water = 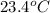
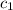 = specific heat of aluminium =
= specific heat of aluminium = 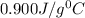
 = specific heat of water=
= specific heat of water= 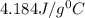
![-12.1\times 0.900\times (24.9-81.7)=-[m_2\times 4.184\times (24.9-23.4)]](/tpl/images/0500/2981/9ba9b.png)