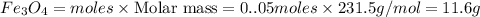Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8....

Chemistry, 26.01.2021 01:10 lazavionadams81
Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8.4 g of Fe, how many of Fe 3O4 are formed?
a) Calculate the limiting reactant
b) Calculate the number of grams of Al produced.
c) Calculate the number of grams of Fe3O4 produced.
d) Calculate the percent yield if 10g of Fe O4 were obtained?

Answers: 3
Another question on Chemistry




Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Questions


Mathematics, 03.05.2021 16:10


Mathematics, 03.05.2021 16:10




Mathematics, 03.05.2021 16:10



Mathematics, 03.05.2021 16:10

Chemistry, 03.05.2021 16:10



Mathematics, 03.05.2021 16:10


Social Studies, 03.05.2021 16:10



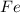 is the limiting reagent
is the limiting reagent 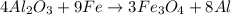
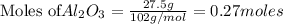
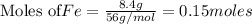
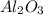
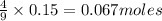 of
of 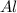
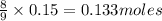 of
of 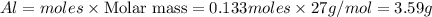
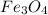
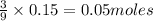 of
of