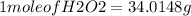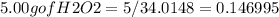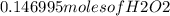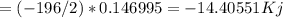Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l)...

Chemistry, 19.01.2020 09:31 calvinclifton
Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l) 2h2o(l) + o2(g) enthalpy=-196kj
calculate the value of q when 5.00g of h20(l) decomposes at constant pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Questions

Mathematics, 15.06.2021 22:40



English, 15.06.2021 22:40




Mathematics, 15.06.2021 22:40

Mathematics, 15.06.2021 22:40


English, 15.06.2021 22:40

Mathematics, 15.06.2021 22:40

Mathematics, 15.06.2021 22:40



Mathematics, 15.06.2021 22:40


Social Studies, 15.06.2021 22:40

English, 15.06.2021 22:40

English, 15.06.2021 22:40