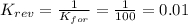I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure would
N2(g)+3H2(g)⇄2NH3(g)
a. increase [H2]
b. increase [NH3]
c. increase [N2]
d. have no effect
2. If K=100, then the value of K for the reverse reaction is
a. the same value
b. can only be determined by experimentation
c. the negative of the value for the forward reaction
d. 0.01

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure woul...
Questions

Geography, 20.09.2019 05:00








History, 20.09.2019 05:00

Mathematics, 20.09.2019 05:00

English, 20.09.2019 05:00


Health, 20.09.2019 05:00

Mathematics, 20.09.2019 05:00

Mathematics, 20.09.2019 05:00



Physics, 20.09.2019 05:00

Chemistry, 20.09.2019 05:00

Mathematics, 20.09.2019 05:00