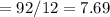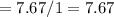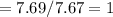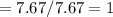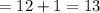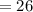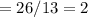Chemistry, 01.02.2021 21:20 kkennethbrown9222
6.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 26. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured:
product mass
carbon dioxide 20.31g
water 4.16 g
Use this information to find the molecular formula of X

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2


Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
You know the right answer?
6.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have...
Questions


Mathematics, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20


History, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20

Geography, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20

English, 27.10.2020 07:20

History, 27.10.2020 07:20

History, 27.10.2020 07:20

Biology, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20

Health, 27.10.2020 07:20

Physics, 27.10.2020 07:20

Computers and Technology, 27.10.2020 07:20

English, 27.10.2020 07:20


Mathematics, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20

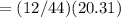 grams
grams 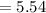 gram
gram
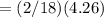 grams
grams 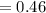 gram
gram
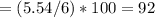 %
%
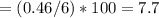 %
%