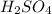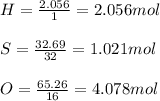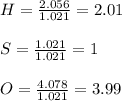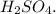Chemistry, 02.02.2021 14:00 adamkinney6110
A compound was analyzed and found to contain the following percent composition: 2.056% hydrogen, 32.69% S, and 65.26% oxygen. Calculate the empirical formula.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
A compound was analyzed and found to contain the following percent composition: 2.056% hydrogen, 32....
Questions


Mathematics, 04.09.2020 08:01


Mathematics, 04.09.2020 08:01


History, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01


Chemistry, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Chemistry, 04.09.2020 08:01

Chemistry, 04.09.2020 08:01

Law, 04.09.2020 08:01

Health, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Computers and Technology, 04.09.2020 08:01


Arts, 04.09.2020 08:01

English, 04.09.2020 08:01