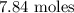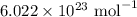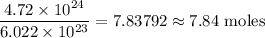Chemistry, 08.02.2021 09:00 smarty5187
If you have 4.72 x 10^24 atoms of Carbon, how many moles of Carbon do you have?
Question 5 options:
2.84 x 10^48 moles of carbon
4.12 x 10^24 moles of carbon
4.72 x 10^24 moles of carbon
7.84 moles of carbon

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 23.06.2019 11:50
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon
Answers: 1
You know the right answer?
If you have 4.72 x 10^24 atoms of Carbon, how many moles of Carbon do you have?
Question 5 options:...
Questions

















Mathematics, 16.11.2019 03:31


Mathematics, 16.11.2019 03:31