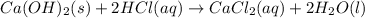Chemistry, 11.02.2021 23:20 babygirl123468
A sample of 7.34 g of solid calcium hydroxide is added to 34.5 mL of 0.380 M Aqueous hydrochloric acid. Write the balanced chemical equation for the reaction. Physical states are optional.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
A sample of 7.34 g of solid calcium hydroxide is added to 34.5 mL of 0.380 M Aqueous hydrochloric ac...
Questions

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

English, 11.02.2021 14:00

Biology, 11.02.2021 14:00

Physics, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00


Chemistry, 11.02.2021 14:00


Spanish, 11.02.2021 14:00

History, 11.02.2021 14:00

Geography, 11.02.2021 14:00



History, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00


Physics, 11.02.2021 14:00