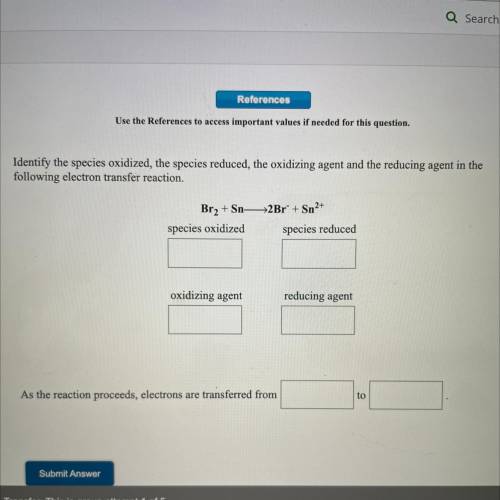
Br2 + Sn -> 2Br^- + Sn^2+
species oxidized:
species reduced:
oxidizing agent:
...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Questions

Mathematics, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01

Chemistry, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01

History, 04.10.2020 14:01



English, 04.10.2020 14:01

Social Studies, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01

Spanish, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01



Mathematics, 04.10.2020 14:01