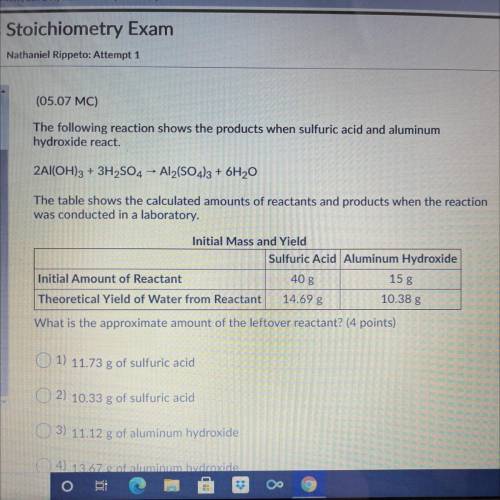
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2A...

The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2Al(OH)3 + 3H2SO4 - Al2(SO4)3 + 6H20
The table shows the calculated amounts of reactants and products when the reaction
was conducted in a laboratory.
What is the approximate amount of the leftover reactant?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Clouds form when water vapor to form small droplets. a. humidifies b. condenses c. evaporates d. precipitates
Answers: 2

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
You know the right answer?
Questions

Mathematics, 24.09.2019 03:10

Mathematics, 24.09.2019 03:10


Social Studies, 24.09.2019 03:10

History, 24.09.2019 03:10

History, 24.09.2019 03:10

Geography, 24.09.2019 03:10



Chemistry, 24.09.2019 03:10





Biology, 24.09.2019 03:20

French, 24.09.2019 03:20



Mathematics, 24.09.2019 03:20




