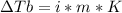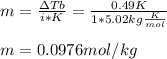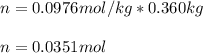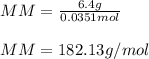Chemistry, 19.02.2021 09:30 marlandwilliams10
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0.49 K higher than the boiling point of pure CC14. Calculate the molar mass of
adrenaline. (K = 5.02 kg K/mol).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0...
Questions

Computers and Technology, 19.10.2021 15:20

Chemistry, 19.10.2021 15:20


Mathematics, 19.10.2021 15:20

Social Studies, 19.10.2021 15:20


Physics, 19.10.2021 15:20

Mathematics, 19.10.2021 15:20


Computers and Technology, 19.10.2021 15:20


Mathematics, 19.10.2021 15:20

History, 19.10.2021 15:20


Chemistry, 19.10.2021 15:30





Mathematics, 19.10.2021 15:30