PLEASE HELP ASAP
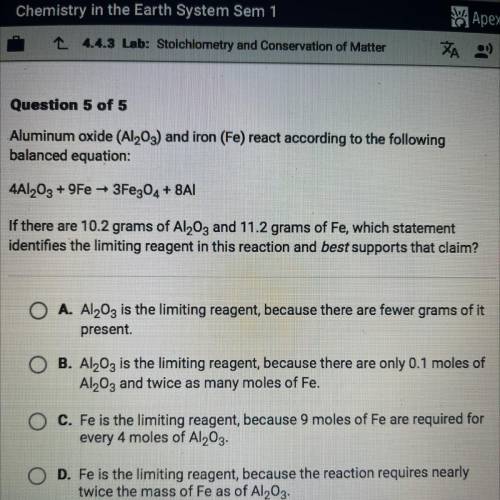
Aluminum oxide (Al2O3) and iron (Fe) react according to the following
balanc...

PLEASE HELP ASAP
Aluminum oxide (Al2O3) and iron (Fe) react according to the following
balanced equation:
4Al2O3 + 9Fe + 3Fe304 + 8A|
If there are 10.2 grams of Al2O3 and 11.2 grams of Fe, which statement
identifies the limiting reagent in this reaction and best supports that claim?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

You know the right answer?
Questions
















Mathematics, 14.11.2019 01:31


Mathematics, 14.11.2019 01:31

French, 14.11.2019 01:31



