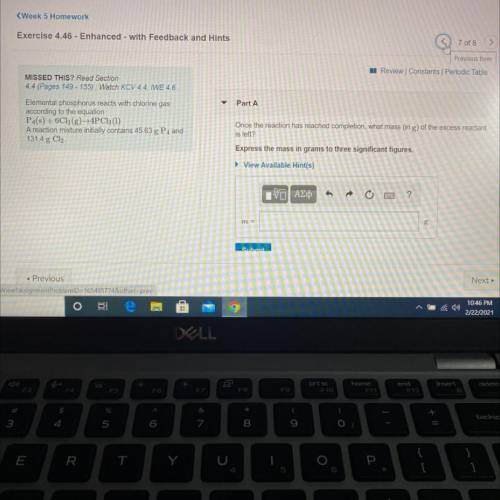
Elemental phosphorus reacts with chlorine gas
according to the equation
P4(s) + 6C12(g)+4PC13...

Chemistry, 23.02.2021 09:00 araminaara691
Elemental phosphorus reacts with chlorine gas
according to the equation
P4(s) + 6C12(g)+4PC13 (1)
A reaction mixture initially contains 45.63 g P4 and
131.4 g Cl.
Once the reaction has reached completion, what mass (in g) of the excess reactant is left?
Express the mass in grams to three significant figures.
please help i’ll give brainliest

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


You know the right answer?
Questions


Mathematics, 22.02.2021 19:10





Mathematics, 22.02.2021 19:10

Mathematics, 22.02.2021 19:10

Mathematics, 22.02.2021 19:10


Computers and Technology, 22.02.2021 19:10

Arts, 22.02.2021 19:10








Mathematics, 22.02.2021 19:10



