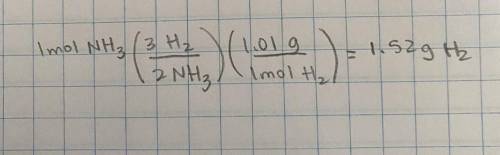Chemistry, 25.02.2021 02:30 kaseykrueger47
In the reaction :N2 + 3H22NH3 how many grams of H2 are needed to produce 1 mole on ammonia

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
In the reaction :N2 + 3H22NH3 how many grams of H2 are needed to produce 1 mole on ammonia...
Questions


Mathematics, 23.04.2021 15:00




Mathematics, 23.04.2021 15:00

Mathematics, 23.04.2021 15:00


Mathematics, 23.04.2021 15:00

Medicine, 23.04.2021 15:00

Business, 23.04.2021 15:00

Mathematics, 23.04.2021 15:00

Mathematics, 23.04.2021 15:00



Social Studies, 23.04.2021 15:00

Mathematics, 23.04.2021 15:00


Mathematics, 23.04.2021 15:00

Mathematics, 23.04.2021 15:00