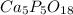Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
A sample of a compound used to polish dentures and as a nutrient and dietary supplement is analyzed...
Questions

Social Studies, 14.10.2019 16:20

History, 14.10.2019 16:20

Geography, 14.10.2019 16:20


Mathematics, 14.10.2019 16:20

English, 14.10.2019 16:20

Physics, 14.10.2019 16:20

History, 14.10.2019 16:20


History, 14.10.2019 16:20

English, 14.10.2019 16:20



History, 14.10.2019 16:20


English, 14.10.2019 16:20




Mathematics, 14.10.2019 16:20