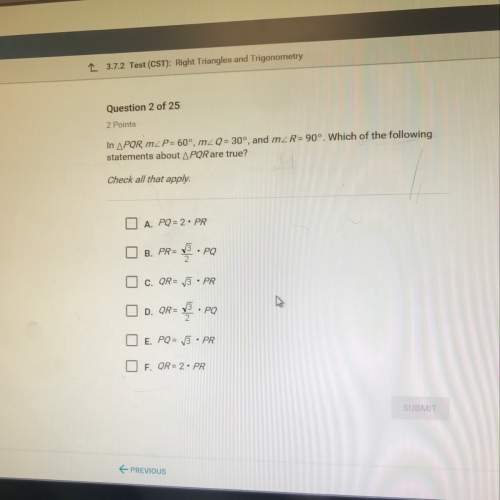
Chemistry, 27.02.2021 23:40 jakeisadog698
The balanced chemical equation represent the reaction between zinc (Zn) and hydrochloric acid (HCI).
Zn + 2HCl + H2 + ZnCl2
At STP, how many liters of hydrogen gas (Hz) will be produced if 31.2 g of zinc is allowed to react with excess
hydrochloric acid?
A 2.82L
B 4.30 L
C 9.66 L
D 10.7 L

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1


Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
The balanced chemical equation represent the reaction between zinc (Zn) and hydrochloric acid (HCI)....
Questions





Mathematics, 27.02.2020 21:58

Mathematics, 27.02.2020 21:58








English, 27.02.2020 21:58

Mathematics, 27.02.2020 21:58


English, 27.02.2020 21:58

Spanish, 27.02.2020 21:58


English, 27.02.2020 21:58