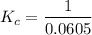Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2Hg (l) + O2 (g) → 2HgO (s)
At a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(II) oxide, mercury, and oxygen at equilibrium has the following composition:
compound amount
Hg 14.7g
O2 13.4g
HgO 17.8g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits. Clears your work. Undoes your last action. Provides information about entering answers.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

You know the right answer?
Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2Hg (l) + O2 (g) → 2HgO (s)
Questions


Mathematics, 15.12.2020 16:30







Mathematics, 15.12.2020 16:30




Mathematics, 15.12.2020 16:30

Mathematics, 15.12.2020 16:30



English, 15.12.2020 16:30

Mathematics, 15.12.2020 16:30


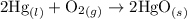
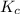 contains aqueous an dgas species only.
contains aqueous an dgas species only. ![$K_c=\frac{1}{[O_2]}$](/tpl/images/1168/7990/eab15.png) ............(1)
............(1)![$[O_2]= \frac{n_{O_2}}{V_{soln}}$](/tpl/images/1168/7990/80b5d.png) ................... (2)
................... (2)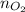 = no. of moles of oxygen gas (mol)
= no. of moles of oxygen gas (mol)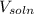 = volume of solution (L)
= volume of solution (L)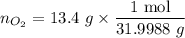
![$[O_2] =\frac{0.418 \ \text{mol}}{6.9 \ \text{L}}$](/tpl/images/1168/7990/d9674.png)