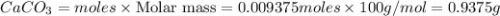Show
your calculations for full marks
1. Calculate the mass of CaCO3(s) required to react wit...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

You know the right answer?
Questions


History, 26.06.2019 13:30

History, 26.06.2019 13:30






Mathematics, 26.06.2019 13:30

Biology, 26.06.2019 13:30



Mathematics, 26.06.2019 13:30


Biology, 26.06.2019 13:30

History, 26.06.2019 13:30

Biology, 26.06.2019 13:30


Physics, 26.06.2019 13:30

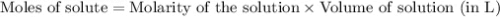 .....(1)
.....(1)
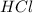 solution = 0.75 M
solution = 0.75 M
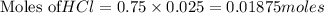
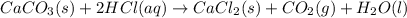
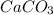
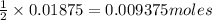 of
of