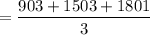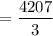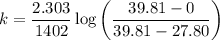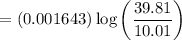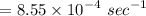3. Methyl acetate is hydrolyzed at 25 oC in acidic environment. Aliquots of equal volume are removed and titrated with NaOH solution. The hydrolysis reaction is irreversible. The results are Time/s 339 1242 2745 4546 infinite Volume of base/ mL 26.34 27.80 29.70 31.81 39.81 Write the reaction of hydrolysis of methyl acetate. (3 pts.) Neglect the reverse reaction. Find the order of the hydrolysis reaction and the value of the rate constant at this temperature. (12 pts.) Total 15 pts.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1


Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
3. Methyl acetate is hydrolyzed at 25 oC in acidic environment. Aliquots of equal volume are removed...
Questions




Computers and Technology, 18.03.2020 01:25


Computers and Technology, 18.03.2020 01:25




Biology, 18.03.2020 01:25

Mathematics, 18.03.2020 01:25


Mathematics, 18.03.2020 01:25

Mathematics, 18.03.2020 01:25

Mathematics, 18.03.2020 01:25

Physics, 18.03.2020 01:25

Physics, 18.03.2020 01:25

English, 18.03.2020 01:25

Biology, 18.03.2020 01:25

Mathematics, 18.03.2020 01:25

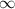 39.81
39.81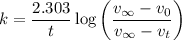
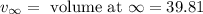
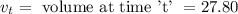
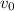 = volume at time 0 = 0
= volume at time 0 = 0