
Chemistry, 11.03.2021 05:30 feliciagraham14
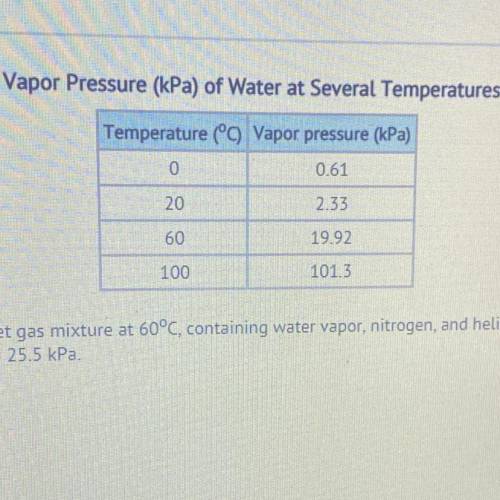
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and helium. The partial pressures are Pnitrogen = 53.0 kPa and Phelium = 25.5 kPa.
A
58.58 kPa
B)
78.50 kPa
C)
98.42 kPa
D
101.32 KP

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and heliu...
Questions















Computers and Technology, 05.09.2019 17:10







