
Chemistry, 12.03.2021 08:30 Conner5459
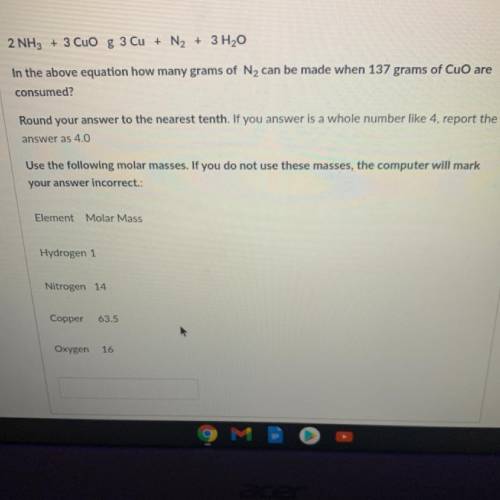
2 NH3 + 3 CuO g 3 Cu + N2 + 3 H2O
In the above equation how many grams of N2 can be made when 137 grams of CuO are
consumed?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1


Chemistry, 23.06.2019 15:50
Many radioactive atoms that have large masses undergo radioactive decay by releasing a particle that is identical to a helium-4 nucleus. what changes in the original atom are expected as a result of this natural phenomenon? the atomic number and the mass number will decrease. the atomic number and the mass number will increase. the atomic number will increase, and the mass number will decrease. the atomic number will decrease, and the mass number will increase.
Answers: 2
You know the right answer?
2 NH3 + 3 CuO g 3 Cu + N2 + 3 H2O
In the above equation how many grams of N2 can be made when 137 g...
Questions






Mathematics, 15.10.2019 18:40




Mathematics, 15.10.2019 18:40


Mathematics, 15.10.2019 18:40

Mathematics, 15.10.2019 18:40

English, 15.10.2019 18:40



History, 15.10.2019 18:40

Mathematics, 15.10.2019 18:40




