1. What is the wavelength (in meters) of a radio wave with a frequency of 9.31 x Hz?
2....

Chemistry, 18.03.2021 02:00 nathaniel12
1. What is the wavelength (in meters) of a radio wave with a frequency of 9.31 x 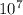 Hz?
Hz?
2. Calculate the energy of:
a) a photon with a wavelength of 5.00 x 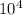 nm.
nm.
b) a photon with a wavelength of 5.00 x 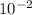 nm.
nm.
3. The energy of a photon is 5.87 x 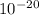 J. What is its wavelength in nm?
J. What is its wavelength in nm?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
You know the right answer?
Questions












Computers and Technology, 18.12.2019 01:31


Computers and Technology, 18.12.2019 01:31








