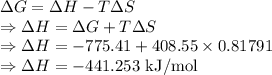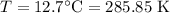Chemistry, 18.03.2021 02:50 Mw3spartan17
For a particular reaction at 135.4 °C, Δ=−775.41 kJ/mol, and Δ=817.91 J/(mol⋅K).
Calculate ΔG for this reaction at 12.7 °C.
Δ=

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
For a particular reaction at 135.4 °C, Δ=−775.41 kJ/mol, and Δ=817.91 J/(mol⋅K).
Calculate ΔG for t...
Questions


Mathematics, 20.05.2021 20:20


Mathematics, 20.05.2021 20:20

Mathematics, 20.05.2021 20:20

Mathematics, 20.05.2021 20:20

Mathematics, 20.05.2021 20:20




History, 20.05.2021 20:20

Chemistry, 20.05.2021 20:20



Social Studies, 20.05.2021 20:20

Arts, 20.05.2021 20:20

Mathematics, 20.05.2021 20:20


Mathematics, 20.05.2021 20:20

Biology, 20.05.2021 20:20

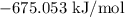
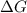 = Gibbs free energy =
= Gibbs free energy = 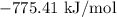
 = Change in entropy =
= Change in entropy = 
 = Temperature =
= Temperature = 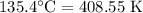
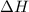 = Change in enthalpy
= Change in enthalpy