
Chemistry, 18.03.2021 02:50 Fireburntbudder
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the temperature is raised to 80.0°C and the pressure increased to 2.40 atm, what is the new volume?
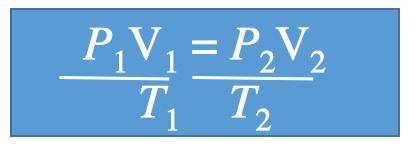

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the tem...
Questions


Mathematics, 06.11.2020 23:40

Chemistry, 06.11.2020 23:40


English, 06.11.2020 23:40


English, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40


Biology, 06.11.2020 23:40



Biology, 06.11.2020 23:40



Chemistry, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40




