Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
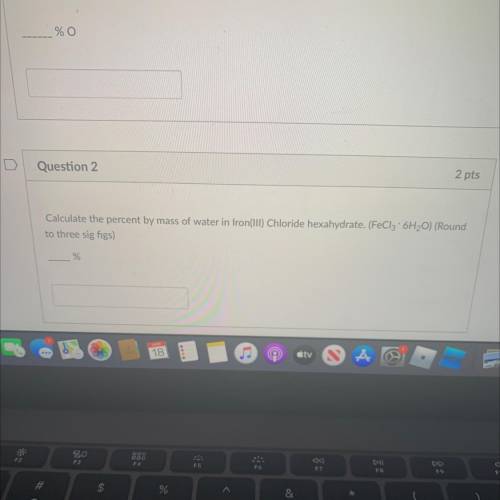
Calculate the percent by mass of water in Iron(III) Chloride hexahydrate. (FeCl3 x 6H2O) (Round
to...
Questions







Health, 24.07.2019 01:30



Health, 24.07.2019 01:30

Health, 24.07.2019 01:30

Health, 24.07.2019 01:30

Health, 24.07.2019 01:30

Health, 24.07.2019 01:30


Health, 24.07.2019 01:30