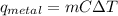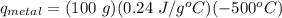Chemistry, 19.03.2021 21:50 momneedshelphmwk
A student investigates a pure metal, X . The student takes a 100.0 g piece of metal X , heats it to 500.0°C , then places it on a 1000.0 g block of ice at 0.0°C . The ice partially melts, and the final temperature of the metal, ice, and melted water is 0.0°C . The student calculates the experimental value of the specific heat capacity of metal X and records it as 0.24 J/(g⋅°C) . Calculate the magnitude of the energy change (qmetal) of metal X during the experiment.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
A student investigates a pure metal, X . The student takes a 100.0 g piece of metal X , heats it to...
Questions

History, 24.10.2019 03:10





English, 24.10.2019 03:10

Chemistry, 24.10.2019 03:10

Geography, 24.10.2019 03:10

Physics, 24.10.2019 03:10




English, 24.10.2019 03:10

Spanish, 24.10.2019 03:10


Mathematics, 24.10.2019 03:10

Mathematics, 24.10.2019 03:10



Biology, 24.10.2019 03:10