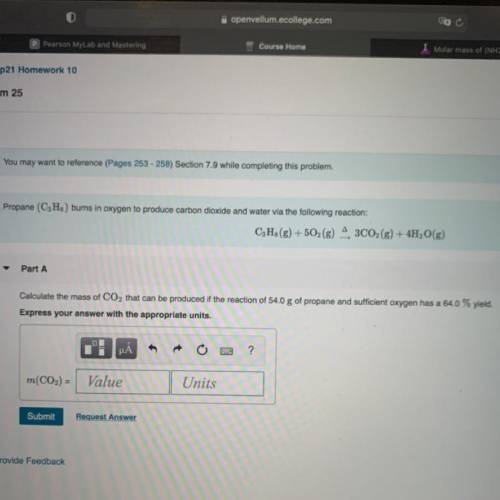
Chemistry, 21.03.2021 06:50 HTKPenguin
Calculate the mass of CO2 that can be produced if the reaction of 54.0 g of propane and sufficient oxygen has a 64.0% yield.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
Calculate the mass of CO2 that can be produced if the reaction of 54.0 g of propane and sufficient o...
Questions





Social Studies, 14.03.2020 02:43


History, 14.03.2020 02:44



Computers and Technology, 14.03.2020 02:44






History, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44