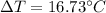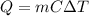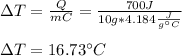Chemistry, 22.03.2021 03:40 damienwoodlin6
Calculate the expected temperature change for 10 grams of water when 700 joules of energy are added.(Enter answer to 2 decimal places.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

You know the right answer?
Calculate the expected temperature change for 10 grams of water when 700 joules of energy are added....
Questions






English, 21.08.2019 19:30


Mathematics, 21.08.2019 19:30


Biology, 21.08.2019 19:30










Geography, 21.08.2019 19:30