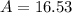There are two isotopes of an unknown element, X-19 and X-21. The abundance of X-19 is 12.01%. Now that you have the contribution from the X-19 isotope (2.282) and from the X-21 isotope (18.48), what is the average atomic mass (in amu) of this element using four significant figures

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3
You know the right answer?
There are two isotopes of an unknown element, X-19 and X-21. The abundance of X-19 is 12.01%. Now th...
Questions

Mathematics, 14.01.2021 20:10



Mathematics, 14.01.2021 20:10




Mathematics, 14.01.2021 20:10



Mathematics, 14.01.2021 20:10

Mathematics, 14.01.2021 20:10

Physics, 14.01.2021 20:10

Mathematics, 14.01.2021 20:10


English, 14.01.2021 20:10

Mathematics, 14.01.2021 20:10

Business, 14.01.2021 20:10

Mathematics, 14.01.2021 20:10

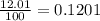
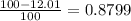
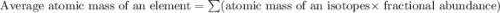
![A=\sum[(2.282\times 0.1201)+(18.48\times 0.8799)]](/tpl/images/1212/1640/eff37.png)