
Chemistry, 24.03.2021 20:40 dounutalien
Which of the following gas samples would contain the same amount of gas as 200 mL of helium, He(g), at 25° C and 1 atm?
Select one:
a. all the above
b. 200 mL of neon, Ne (g), at 25° C and 1 atm
c. 40 mL of methane, CH4 (g), at 25° C and 1 atm
d. 400 mL of hydrogen, H2 (g), at 25° C and 1 atm
Need done asap! Will give 5 stars!

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

You know the right answer?
Which of the following gas samples would contain the same amount of gas as 200 mL of helium, He(g),...
Questions

German, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00


History, 25.11.2021 14:00




Health, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00


Social Studies, 25.11.2021 14:00


History, 25.11.2021 14:00


Computers and Technology, 25.11.2021 14:00


English, 25.11.2021 14:00


Spanish, 25.11.2021 14:00

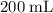 of neon
of neon 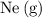 at
at 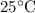 and
and 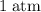 (the second choice) would contain an equal number of gas particles as
(the second choice) would contain an equal number of gas particles as 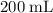 of
of 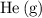 at
at 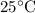 and
and 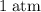 (assuming that all four gas samples behave like ideal gases.)
(assuming that all four gas samples behave like ideal gases.) 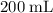 . Hence, that choice would be the only one with as many gas particles as
. Hence, that choice would be the only one with as many gas particles as 

