
Chemistry, 25.03.2021 03:00 lakhanir2013
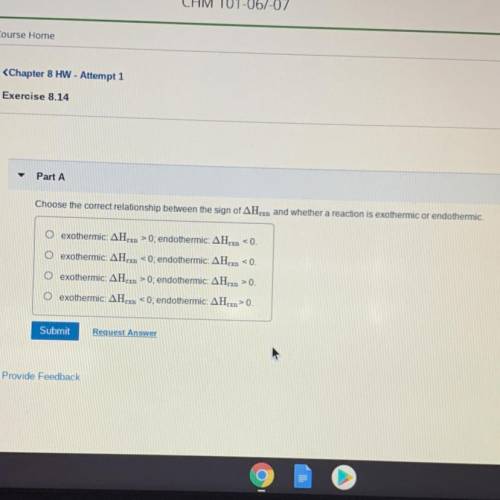
Choose the correct relationship between the sign of delta Hrxn and whether a reaction is exorhermic or endothermic.
•exothermic: delta Hrxn>0; endothermic: delta Hrxn<0.
•exothermic: delta Hrxn<0; endothermic: delta Hrxn<0.
•exothermic: delta Hrxn>0; endothermic: delta Hrxn>0.
•exothermic: delta Hrxn<0; endothermic: delta Hrxn>0.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
Choose the correct relationship between the sign of delta Hrxn and whether a reaction is exorhermi...
Questions


English, 21.08.2019 18:40


Mathematics, 21.08.2019 18:40

Social Studies, 21.08.2019 18:40




Mathematics, 21.08.2019 18:40






Mathematics, 21.08.2019 18:40

Mathematics, 21.08.2019 18:40

Mathematics, 21.08.2019 18:40



Mathematics, 21.08.2019 18:40



