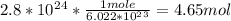How many moles of titanium are in a sample containing 2.8 x 10^24 atoms?
1) 1.69 x 10^48
2) 4...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Questions




Mathematics, 19.11.2020 19:50



Mathematics, 19.11.2020 19:50

History, 19.11.2020 19:50


Social Studies, 19.11.2020 19:50

English, 19.11.2020 19:50

Biology, 19.11.2020 19:50



Biology, 19.11.2020 19:50

English, 19.11.2020 19:50


History, 19.11.2020 19:50