2 attempts left
Check my work
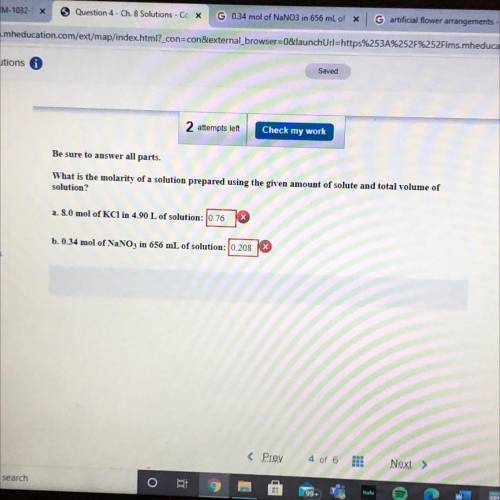
Be sure to answer all parts.
What is the molarity of a so...

Chemistry, 30.03.2021 23:30 princesstn28oqlfir
2 attempts left
Check my work
Be sure to answer all parts.
What is the molarity of a solution prepared using the given amount of solute and total volume of
solution?
a. 8.0 mol of KCl in 4.90 L of solution: 0.76
X
b. 0.34 mol of NaNO3 in 656 mL of solution: 10.208
X

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Questions


Biology, 17.11.2020 18:30



Business, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30


Mathematics, 17.11.2020 18:30







Mathematics, 17.11.2020 18:30


English, 17.11.2020 18:30





