Phase Diagram For. compound
2.5
2
1.5
Pro
40
50 60 700010110
Te...

Phase Diagram For. compound
2.5
2
1.5
Pro
40
50 60 700010110
Tempere)
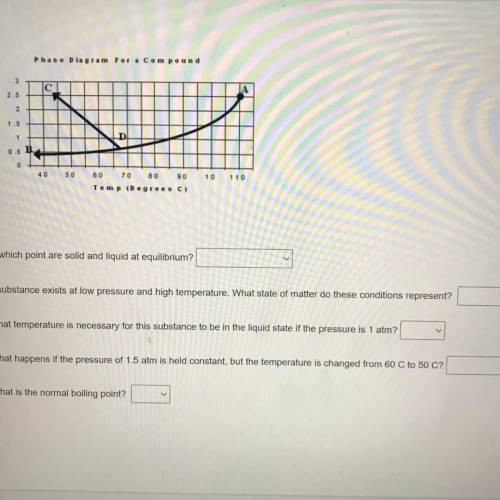
At which point are solid and liquid at equilibrium?
A substance exists at low pressure and high temperature. What state of matter do these conditions represent?
What temperature is necessary for this substance to be in the liquid state if the pressure is 1 atm?
What happens if the pressure of 1.5 atm is held constant, but the temperature is changed from 60 C to 50 C?
What is the normal boiling point?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
Questions


Mathematics, 26.11.2019 18:31















Mathematics, 26.11.2019 18:31





