
Chemistry, 05.04.2021 19:40 softballgirl3589
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0°C, and a pressure of 0.980 atm, and a mass of 0.207 g.
(hint: find moles first and remember that molar mass is the mass per mole”)
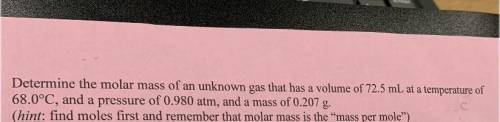

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0...
Questions

Mathematics, 10.12.2020 16:30












Mathematics, 10.12.2020 16:30

Health, 10.12.2020 16:30

Mathematics, 10.12.2020 16:30

Mathematics, 10.12.2020 16:30


Biology, 10.12.2020 16:30




