
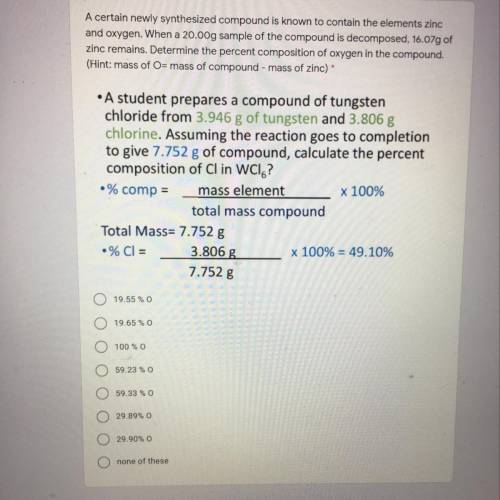
A certain newly synthesized compound is known to contain the elements zinc
and oxygen. When a 20.00g sample of the compound is decomposed, 16.07g of
zinc remains. Determine the percent composition of oxygen in the compound.
(Hint: mass of O= mass of compound - mass of zinc)
Answers are at the bottom someone please help

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
A certain newly synthesized compound is known to contain the elements zinc
and oxygen. When a 20.00...
Questions


Mathematics, 08.06.2021 07:50



Mathematics, 08.06.2021 07:50




Physics, 08.06.2021 07:50




History, 08.06.2021 07:50




English, 08.06.2021 08:00


Mathematics, 08.06.2021 08:00



