
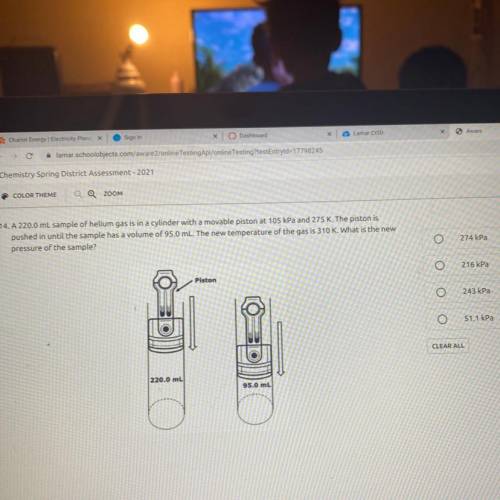
A 220.0 mL sample of helium gas is in a cylinder with a movable piston at 105 kPa and 275 K. The piston is
pushed in until the sample has a volume of 95.0 mL. The new temperature of the gas is 310 K. What is the new
pressure of the sample?
274 kPa
216 kPa
Piston
243 kPa
51.1 kPa

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 11:30
Which of the following is a possible formula unit? (2 points) select one: a. pbo b. li2b c. al2pb3 d. clo
Answers: 1

Chemistry, 23.06.2019 15:30
Amole, which is a unit in chemistry, contains 6.02 x 1023 atoms or particles. how many zeroes follow the 2 when this number is written out in standard form? a) 21 b) 23 c) 24 d) 25
Answers: 1
You know the right answer?
A 220.0 mL sample of helium gas is in a cylinder with a movable piston at 105 kPa and 275 K. The pis...
Questions

Mathematics, 12.07.2019 21:30

Mathematics, 12.07.2019 21:30


Mathematics, 12.07.2019 21:30


History, 12.07.2019 21:30


Mathematics, 12.07.2019 21:30




Biology, 12.07.2019 21:30




English, 12.07.2019 21:30

History, 12.07.2019 21:30

Computers and Technology, 12.07.2019 21:30


Mathematics, 12.07.2019 21:30



