
Chemistry, 16.04.2021 17:50 ayoismeisalex
A 108.9 g sample of water absorbs 114.6 calories of heat. The specific heat capacity of water is 1 cal/(g·°C). By how much did the temperature of this sample change, in degrees Celsius?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
A 108.9 g sample of water absorbs 114.6 calories of heat. The specific heat capacity of water is 1 c...
Questions

Mathematics, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00

English, 06.01.2021 14:00

Chemistry, 06.01.2021 14:00


Computers and Technology, 06.01.2021 14:00

English, 06.01.2021 14:00

Health, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00



Mathematics, 06.01.2021 14:00

English, 06.01.2021 14:00


Mathematics, 06.01.2021 14:00



Business, 06.01.2021 14:00

English, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00

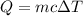
 is the change in temperature
is the change in temperature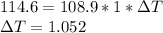 degree Celsius
degree Celsius

